Use a Fischer Projection to Describe the Stereochemistry of
Use the hydroxyl with the bond pointed toward the carbon atom. To form a Fischer projection orient the molecule in your head if not on paper so that the vertical bonds are directed below the plane of the page and the horizontal bonds are directed above the plane of the page.
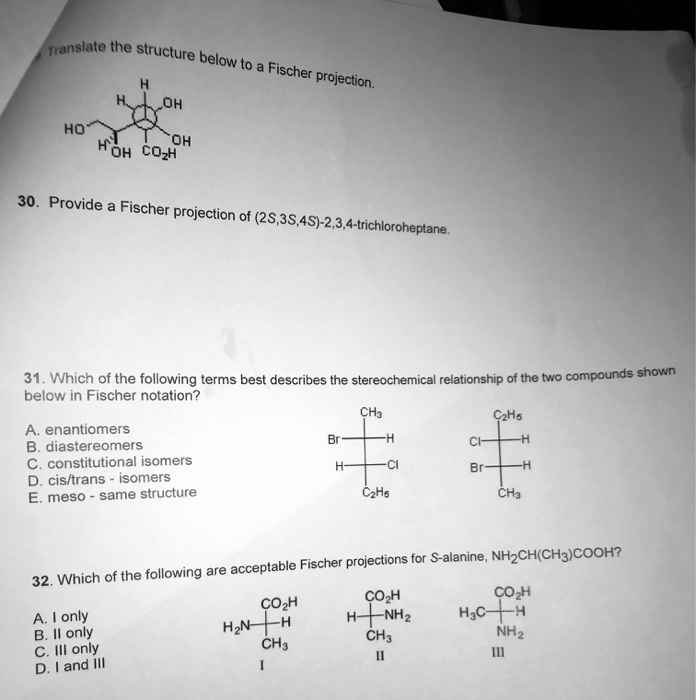
Solved Translate The Structure Below Fischer Projection Oh Ho Koh Oh Cozh 30 Provide Fischer Projection Of 28 38 45 2 3 4 Trichloroheptane 31 Which Of The Following Terms Best Describes The Stereochemical Relationship Of
How are Fischer Projections Drawn.
. One approach is the Fischer projection which represents chiral molecules using a simple cross structure. Its enantiomer --glyceraldehyde was. 4 3 1 2 4 3 2 1 123 - Clockwise R.
Fischer projections can be used to assign stereochemistry. Fischer Projections are abbreviated structural forms that allow one to convey valuable stereochemical information to a chemist or biochemist without them having to draw a more detailed 3D structural representation of the molecule. Use Fischer projections to show the stereochemistry of D- and L-amino acids.
The answers in addition to providing solutions to. The 191 problems in this book cover most of the area of stereochemistry including nomenclature stereogenic elements centers axes planes and their descriptors symmetry inorganic stereochemistry determination of enantiomer excess conformation of acyclic and cyclic compounds and more. Use a Fischer projection to describe the stereochemistry of R-2-dhkorobutane by dragging the functional groups to the appropriate box in the figure.
Use a Fischer projection to describe the stereochemistry of meso-tartaric acid by dragging the hydrogens and hydroxyl groups to the appropriate boxes in the figure below. The two names describe the same molecule which also happens to be a meso compound because it contains a plane of symmetry. The horizontal line represents bonds extending out of the plane of the page whereas the vertical line represents bonds extending into the plane of the page.
Determine the direction of the arrow. Who are the experts. The vertical lines represent bonds directed towards the observer in front of the plane and behind the plane top is behind and bottom is infront the.
Draw the horizontal bonds as wedge lines. Fischer projections are generally used for depicting monosaccharide and amino acids. Use the hydroxyl with the bond pointed toward the carbon atom.
Using a Fischer projection you should be mentally assume the 3D nature of the molecule but to save time they are often omitted. COOH H- -OH HO- - TH COOH HO- -OH H. The precise stereochemistry of monosaccharides defines their biology 910.
Up to 256 cash back Use a Fischer projection to describe the stereochemistry of R-2-chlorobutane by dragging the functional groups to the appropriate box. All monosaccharides are relatively similar and have different orientations along the stereocenter. The effect on the physical or biological properties.
They are still there but we omit them to save time drawing so many structures. Illustrate the listed amino acids just like the given example. Assign the priorities of the four groups.
Stereogenic centres depicted in Fischer projections as either D or L. Use a Fischer projection to describe the stereochemistry of meso-tartaric acid by dragging the hydrogens and hydroxyl groups to the appropriate boxes in the figure below. Use the hydroxyl with the bond pointed toward the carbon atom.
This property is called chirality. These representations are only used for molecules that contain. It has a D-configuration.
Fischer projections despite being two-dimensional structures preserve information about the stereochemistry of molecules and despite not being a representation of how molecules might appear in solution are still widely used by biochemists to specify the stereochemistry of amino acids carbohydrates nucleic acids triterpenoids performance enhancing drugs and. Notice that the aldehyde group has a higher priority than the alcohol because the CO double is counted as if the carbon is connected to two oxygen atoms. Learn vocabulary terms and more with flashcards games and other study tools.
If the lowest priority is pointing away from. If the 4th lowest priority group is vertical the other three groups will show clockwise R or counterclockwise S rotation. Up to 24 cash back University of California Davis For use in UCDavis Chemistry 8118 Series 16 Assigning Stereochemistry using Fischer Projections.
For example glucose can be converted to mannose by the inversion of only one stereochemical centerHistorically the configuration of carbohydrates has been defined by comparison with the most simple sugar glyceraldehyde that contains an aldehyde secondary hydroxyl and a primary hydroxyl at. This Fischer projection represents S-2-chlorobutane. Stereochemistry includes method for determining and describing these relationships.
Use a Fischer projection to describe the stereochemistry of R-2-chlorobutane by dragging the functional groups to the appropriate box in the figure below. Use the hydroxyl with the bond pointed toward the carbon atom. Use a Fischer projection to describe the stereochemistry of R-2-chlorobutane by dragging the functional groups to the appropriate box in the figure below.
In a Fischer projection vertical bonds project away from the viewer and. Similarly to how bond line structures are draw where the hydrogen atoms are omitted. As a simple cross with the stereogenic carbon atom in the centre.
Just like how your left foot doesnt quite fit your right shoe molecules also can have properties that depend on their handedness. We review their content and use your feedback to keep the quality high. Higher molecular weight groups have higher priority.
They are suitable for representing monosaccharide because they have numerous stereocenters or carbons with four unique bonds. The Fischer rules for showing. Fischer projection provide an easy way to draw three dimensional molecule on two dimensional paper and all the bonds are drawn as solid lines around asymmetric carbon atom.
An alternative way to represent stereochemistry is the Fischer Projection which was first used by the German chemist Emil Fischer. Totally arbitrarily -glyceraldehyde was defined as being D because the OH group attached to the C-2 is on the right hand side RHS of the molecules when drawn in its correct Fischer projection in which the CHO or most highly oxidised group appears at the top. Use a Fischer projection to describe the stereochemistry of meso-tartaric acid by dragging the hydrogens and hydroxyl groups to the appropriate boxes in the figure below.
If amino group is on the left of Fischer projection it has a L. The Fischer Projection represents every stereocenter as a cross. Experts are tested by Chegg as specialists in their subject area.
We will go over what makes a molecule chiral stereoisomers assigning configurations using the RS system optical activity and Fischer projections.
Organic Chemistry Stereochemistry Fischer Projections Sparknotes

Fischer Projections In Organic Chemistry Definition Examples Interpretation Video Lesson Transcript Study Com
Fischer Projection Organic Chemistry Video Clutch Prep

Fischer Projection Definition Illustration And Examples

Drawing Fischer Projections In Organic Chemistry Youtube
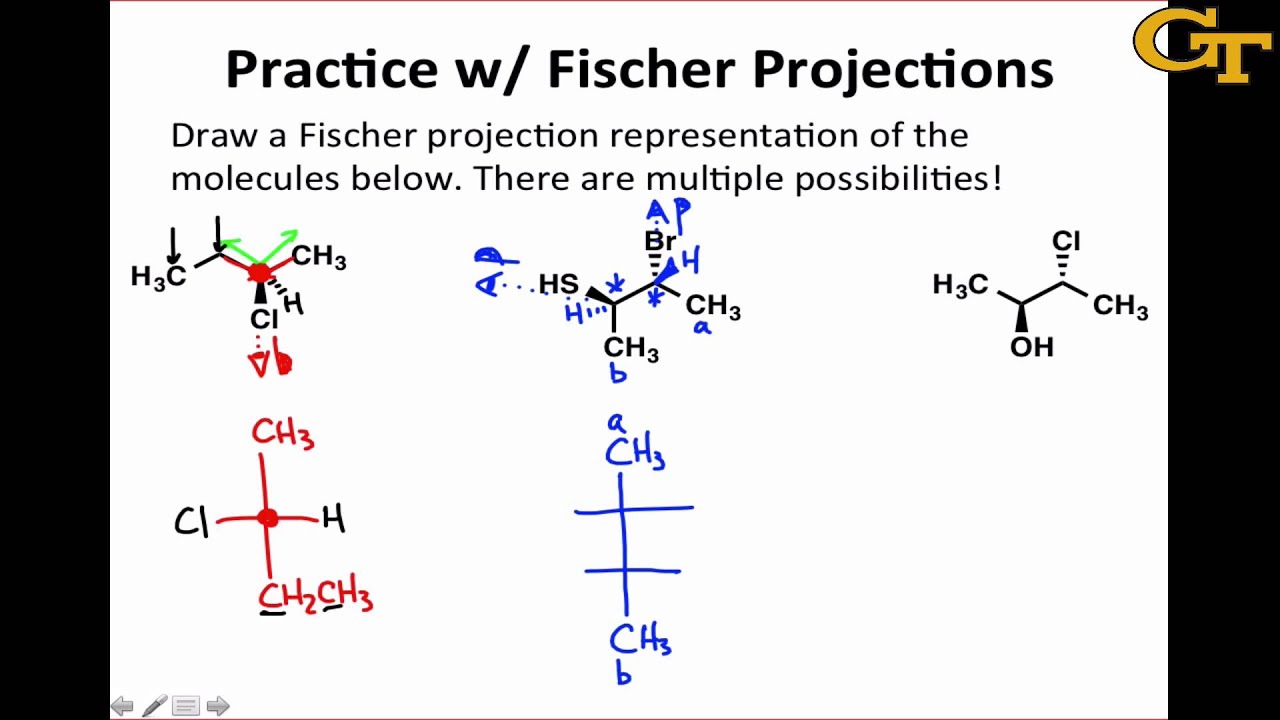
Practice With Fischer Projections Youtube

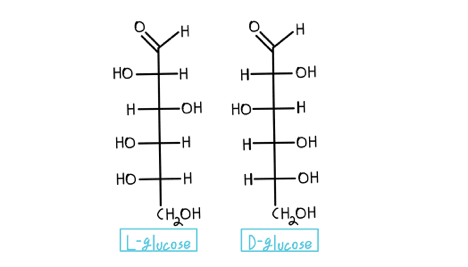
Fischer Projection And Four Hexose Examples From The Left D Glucose Download Scientific Diagram

R And S Configuration On Fischer Projections Chemistry Steps
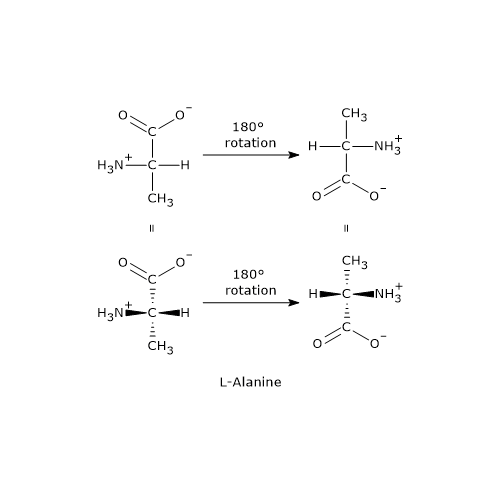
Fischer Projections Or Fischer Projection Formulas

Fischer Projections In Organic Chemistry Rules Examples Interpretation Video Lesson Transcript Study Com

Oneclass Use A Fischer Projection To Describe The Stereochemistry Of R 2 Chlorobutane By Dragging
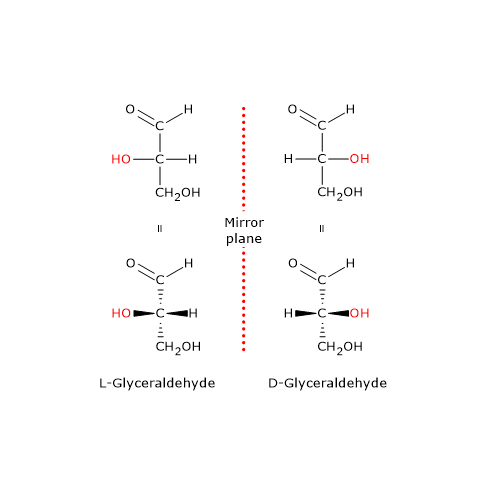
Fischer Rosanoff Convention Or D L System Tuscany Diet
Solved Use Fischer Projections To Show The Stereochemistry Of D And Course Hero
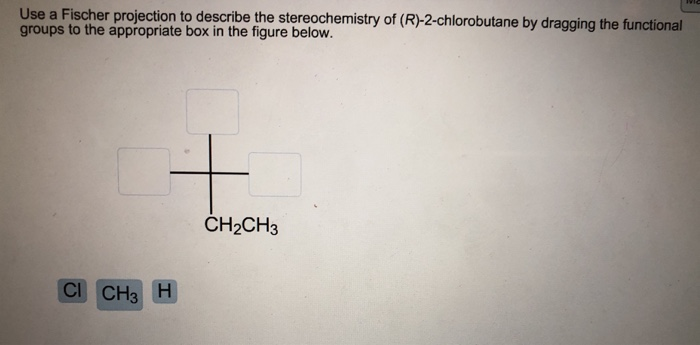
Solved Use A Fischer Projection To Describe The Chegg Com
The Fischer Projection Chemgapedia

Stereochemistry Determining R S Notation For Fischer Projections Youtube

R And S Configuration On Fischer Projections Chemistry Steps

Fischer Projection Definition Illustration And Examples
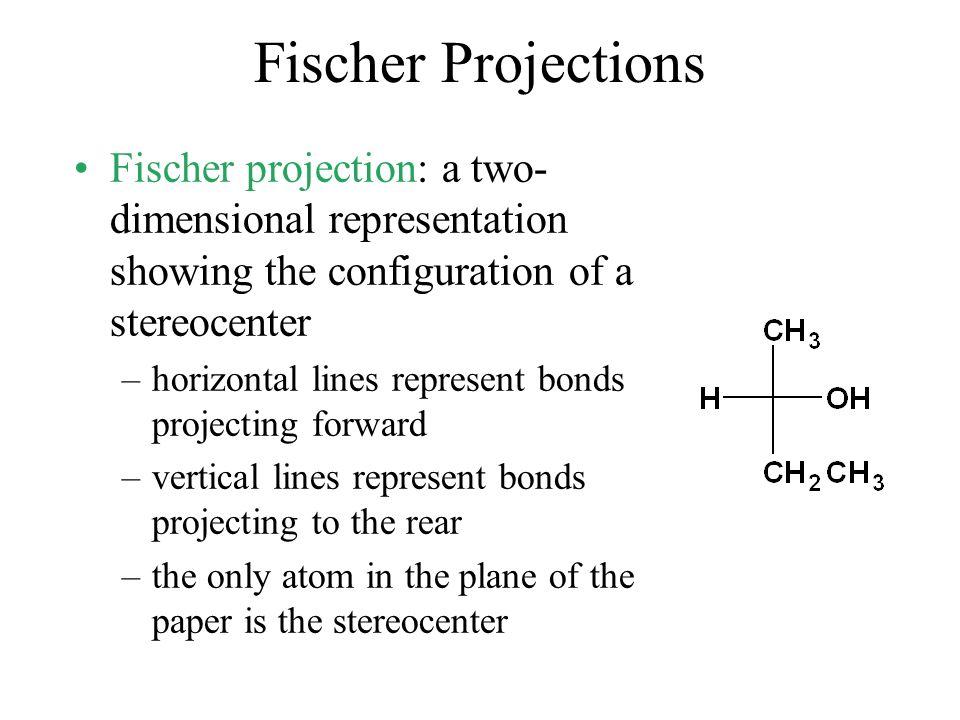
Fischer Projections Fischer Projection A Two Dimensional Representation Showing The Configuration Of A Stereocenter Horizontal Lines Represent Bonds Ppt Download
Comments
Post a Comment